PI: Laurent Catoire, Karine Moncoq
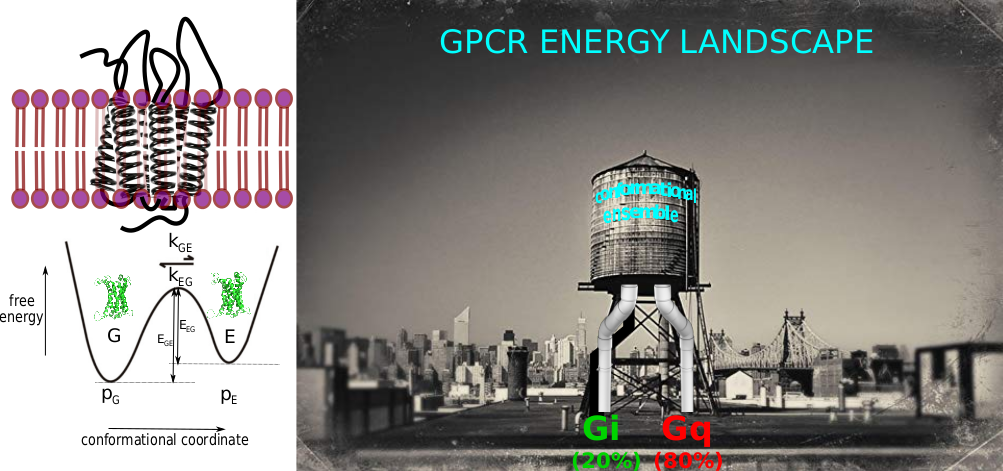
The majority of hormones and neurotransmitters communicate information to cells via G protein-coupled receptors (GPCRs). The large number of biological functions they control also makes these membrane receptors one of the most prominent families of pharmacological targets in biomedicine. GPCRs are considered as flexible and dynamic proteins that can visit multiple conformational states linked to distinct functional outcomes. Despite a tremendous number of structures at the atomic scale that are routinely published, these pictures need to be completed to really get into the function of these complex allosteric machines. The project aims at better characterizing the energy landscape of these receptors by state-of-the art biophysical methods: NMR (kinetic barriers and conformational ensembles), cryo-electron microscopy (structural investigations & conformational ensembles) and single molecule force spectroscopy (kinetic of interactions between ligands and/or effectors with receptors).
Louet et al. (2021) Concerted conformational dynamics andwater movements in the ghrelin G protein-coupled receptor. Elife, 10, e63201.
Damian et al. (2021) Allosteric modulation of ghrelin receptor signaling by lipids. Nat Commun. 12(1):3938. doi: 10.1038/s41467-021-23756-y.
Giusti et al. (2020), Structure of the agonist 12-HHT in its BLT2 receptor-bound state. Sci Rep. 10(1):2630. doi: 10.1038/s41598-020-59571-6.
Casiraghi et al. (2019), NMR analysis of GPCR conformational landscapes and dynamics. Mol Cell Endocrinol. 484:69-77. doi: 10.1016/j.mce.2018.12.019.
Casiraghi et al. (2018) Illuminating the Energy Landscape of GPCRs: The Key Contribution of Solution-State NMR Associated with Escherichia coli as an Expression Host. Biochemistry 57(16):2297-2307. doi: 10.1021/acs.biochem.8b00035.
Chipot et al. (2018) Perturbations of Native Membrane Protein Structure in Alkyl Phosphocholine Detergents: A Critical Assessment of NMR and Biophysical Studies. Chem Rev. 118(7):3559-3607. doi: 10.1021/acs.chemrev.7b00570.
Casiraghi et al. (2016) Functional Modulation of a G Protein-Coupled Receptor Conformational Landscape in a Lipid Bilayer. J Am Chem Soc. 138(35):11170-5. doi: 10.1021/jacs.6b04432
See for more information on the theme: