
Karine Moncoq
UPC MCU

Elodie
Point
Engineer-Assitant
G protein-coupled receptors (GPCRs) constitute the largest integral membrane protein family in the human genome and are involved in many important signal transduction processes. Due to their central role in human physiology, they are associated with a multitude of diseases that make members of this family major pharmacological target. Recently, structural biology of GPCRs has exploded with more than 33 distinct receptors crystallized in different states (http://cherezov.usc.edu/gpcrs.htm). This success results from three major strategies: i) insertion of the T4 lysozyme (T4L) partner stabilizing the GPCR and increasing crystal contacts, ii) the use of lipid phases for crystallization and iii) co-crystallization with strong antagonist.
We are investigating theses approaches to obtain high-resolution crystal structures for the leukotriene B4 receptor BLT1 (figure 1a). To this end, we engineered a modified BLT1 receptor. Positions to insert the T4L sequence either at the N-terminus (T4L-BLT1) or in the third intracellular loop (BLT1-T4L) were selected on the basis of the recent crystal structures of GPCRs providing solid templates. The T4L fusion BLT receptors have been expressed in E.coli as inclusion bodies and successfully purified using the protocol for the unmodified receptor [1] and folded back to its native state thanks to an original amphipol-assisted in vitro folding procedure [2, 3].
The crystallization in presence of antagonists is then performed using the lipid cubic phase (LCP) or in meso method [4]. LCP is a highly viscous bicontinous lipidic liquid crystalline phase providing a more stable native-like membrane environment for crystallization of membrane proteins. This robust approach for crystallizing membrane proteins is lately under development in the laboratory thanks to the arrival of automatic mesophase dispensing robots making LCP crystallogenesis more accessible (figure 1b, crystallization platform link).
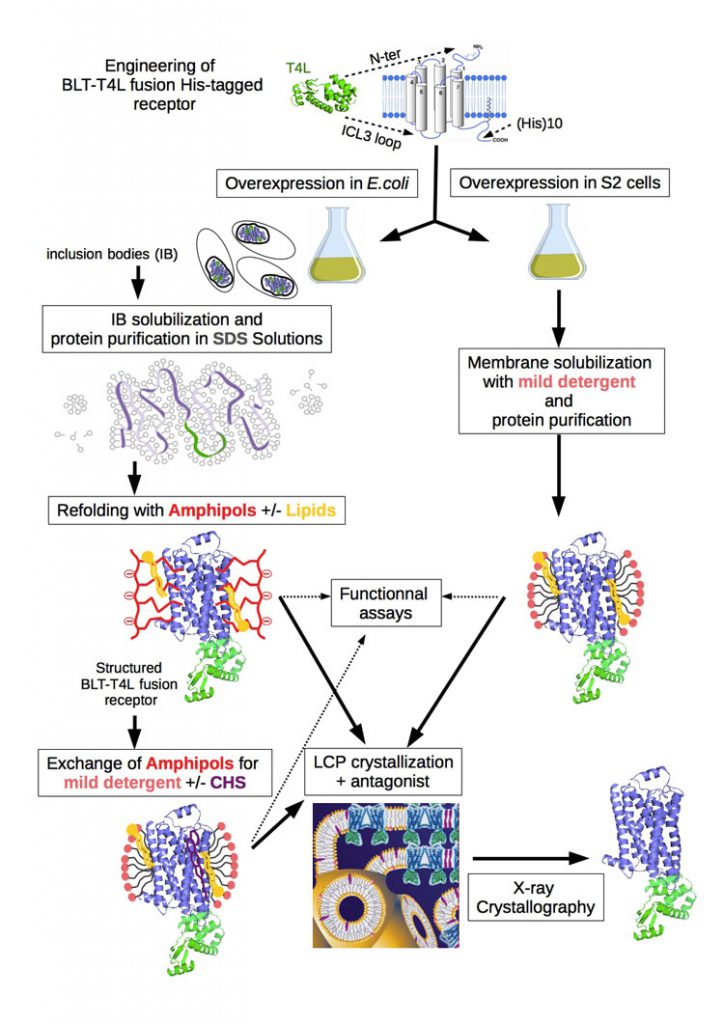
Figure 1 a : General strategy used for high-resolution structure determination of BLT receptors.
Adapted from [3] with cartoon representation of lipid cubic mesophase from [4]. Lipid (yellow), amphipols (red), detergent (light pink), cholesterol or cholesterol hemisuccinate CHS (purple), protein (blue and green; 2-adrenergic GPCR-T4 lysozyme respectively; PDB 2rh1), bilayer and aqueous channels (dark blue).
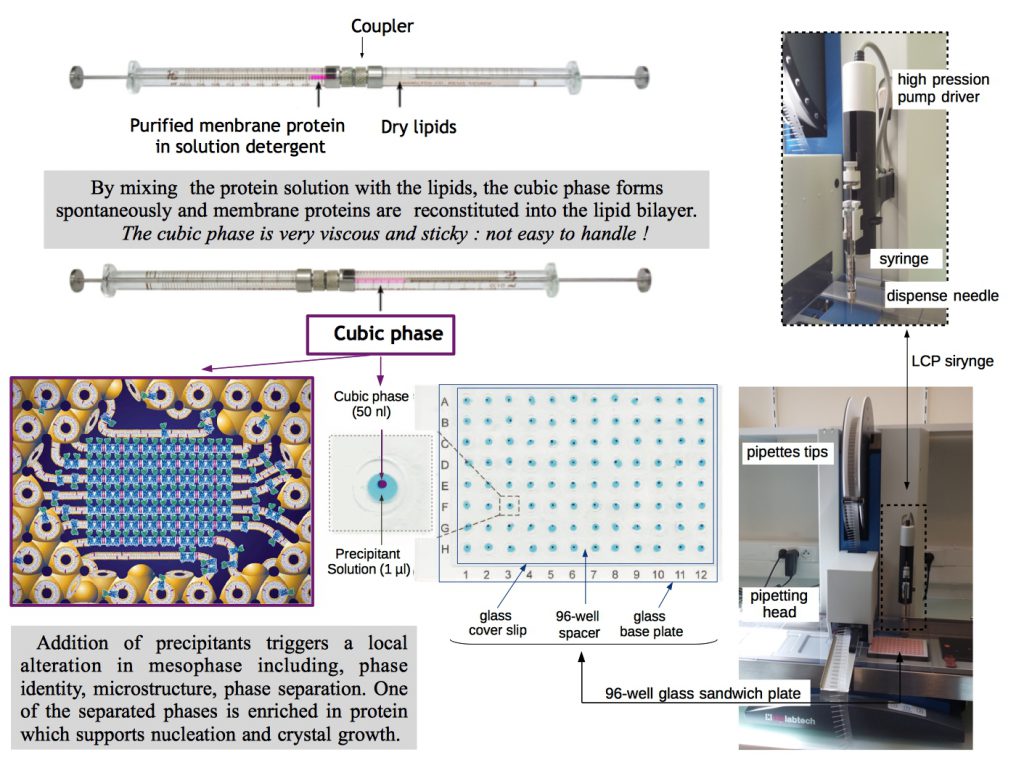
Figure 1 b: Setting up an automated LCP crystallization trial.
Cartoon representation of lipid cubic mesophase and sandwich glass plate illustration were taken from [4] and [5] respectively.
It should be noted that almost all GPCRs actually crystallized have been produced in the sf9 insect cells, for these reason, we plan to express in parallel our constructs in Drosophila S2 cells. Although not yet fully explored, S2 insect cells, appear as a valuable alternative to mammalian cell lines or other virus-infected insect cell systems as sf9 cells. Several major physiological and bioprocess advantages such as the high cell density growth without CO2 supplementation, growth in suspension, use of inducible promoters and stable lines amenable to growth in biorectors for large-scale expression, make S2 cells system a highly potential cellular tool for mass production of GPCRs.
High-resolution X-ray crystallography will provide a structural framework of BLT receptors to understand the observed conformational changes in NMR experiments and should contribute significantly to full understanding of receptor functioning.
Bibliography (in blue from the lab):
- Catoire et al. (2010) Structure of a GPCR ligand in its receptor-bound state: leukotriene B4 adopts a highly constrained conformation when associated to human BLT2. J Am Chem Soc, 132, 9049-57 (Pubmed)
- Dahmane et al. (2009) Amphipol-assisted in vitro folding of G protein-coupled receptors Biochemistry, 48, 6516–21 (Pubmed)
- Casiraghi et al. (2016) Functional modulation of a GPCR conformational landscape in a lipid bilayer. J Am Chem Soc, 138, 11170-5 (Pubmed)
- Caffrey & Cherezov (2009). Crystallizing Membrane Proteins Using Lipidic Mesophases. Nat Protoc, 4, 706–731 (Pubmed)
- Cherezov et al. (2004). A robotic system for crystallizing membrane and soluble proteins in lipidic mesophases. Acta Cryst, D60, 1795-1807 (Pubmed)