PI: Martin Picard, Manuela Zoonens
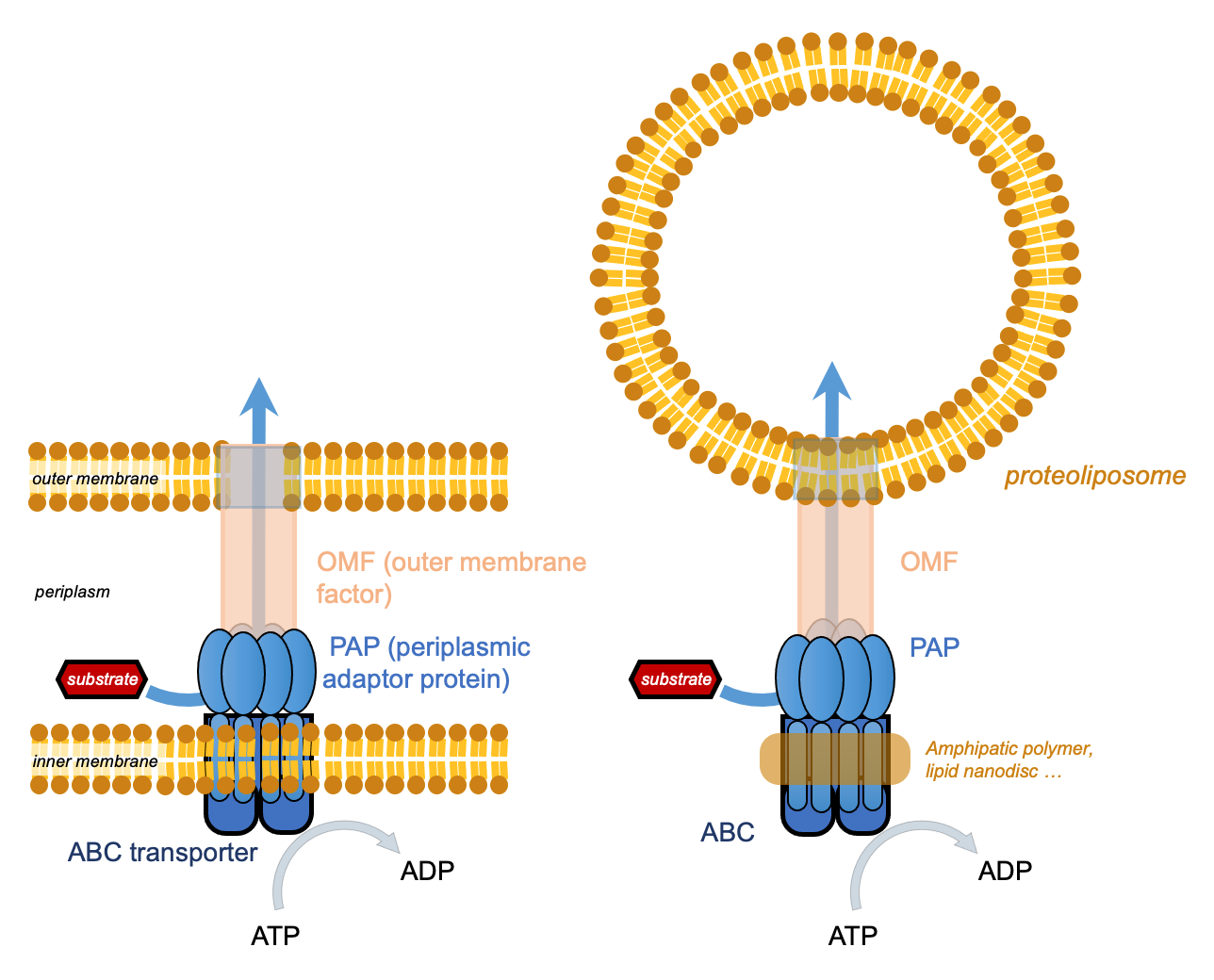
Our laboratory has acknowledged experience in the manipulation and characterization of membrane protein transporters in vitro after solubilization and stabilization by classical or alternative surfactants or after reconstitution into biomimetic systems. We address the problem of antibiotic resistance by focusing on the structure-function analysis of efflux pumps in Gram-negative bacteria. Efflux mechanisms are major determinants of bacterial multidrug resistance, rendering certain antibiotics clinically ineffective by actively exporting them out of the cell before they reach their targets. In Gram-negative bacteria, efflux pumps are organized as tripartite macromolecular machineries assembled within the two membranes of the bacteria. Recent high-resolution structures have revealed the mutual interactions between the pump partners, but many questions remain open about the assembly mechanism of the whole complex and the coupling between energy consumption and substrate transport. The post-doctoral project concerns efflux pumps of the ABC (ATP-binding cassette) superfamily, of which MacAB TolC from Escherichia coli is the most studied member.
We want to understand the role of the membrane environment on the structure and function of this type of pump. Applicants for this position should propose a project using an integrated structure-function study to address this question, by combining cryo-EM in isolated particles on protein complexes stabilized in nanodiscs or solubilized with amphipathic polymers, and in vitro assays. In addition, the project may benefit from an analytical platform for structural biology composed of an array of equipment such as electron microscopy, X-ray crystallography, liquid and solid state nuclear magnetic resonance, small angle X-ray scattering and structural mass spectrometry.
Verchère, A., et al. In vitro transport activity of the fully assembled MexAB-OprM efflux pump from Pseudomonas aeruginosa. Nat. Commun. 6, 6890 (2015).
Daury, L. et al. Tripartite assembly of RND multidrug efflux pumps. Nat. Commun. 7, 10731 (2016).
Glavier, M. et al. Antibiotic export by MexB multidrug efflux transporter is allosterically controlled by a MexA-OprM chaperone-like complex. Nat. Commun. 11, 4948 (2020).
Souabni, H. et al. Quantitative real-time analysis of the efflux by the MacAB-TolC tripartite efflux pump clarifies the role of ATP hydrolysis within mechanotransmission mechanism. Commun. Biol. 10 doi:10.1038/s42003-021-01997-3.
Marconnet, A. et al. Solubilization and Stabilization of Membrane Proteins by Cycloalkane-Modified Amphiphilic Polymers. Biomacromolecules acs.biomac.0c00929 (2020) doi:10.1021/acs.biomac.0c00929
See for more information on the theme: