Until 2023, Head of Laboratory of Physical and Chemical Biology of Membrane Proteins UMR7099 CNRS University Paris-Diderot, Paris, France.
The laboratory is worldwide known for the atomic structure of the membrane photosynthetic complex b6f and for the development by Jean Luc Popot and co-workers of amphipathic polymers, named Amphipols, that keep membrane proteins in solution [1].
I have introduced two new subjects within the unit:
1. The mitochondrial uncoupling protein UCPs with the long term objective to address the fundamental question of how do they transport protons.
2. The second project is about bio-production of membrane proteins, a subject that I initiated in John Walker laboratory (Cambridge, UK see below). Our long term goal is to design a adapted bacterial host for large scale membrane proliferation and membrane protein production.
Group leader in Daniel Ricquier’s laboratory, Hopital Necker, Paris , France 1997-2009
Ricquier’s lab discovered the mitochondrial uncoupling 2 gene and was the first construct Ucp2 knock out mice [2]. Later on, I have shown that UCP2 modulate reactive oxygen production and promote fatty acid oxidation. Human genetic studies and transgenic mice model pointed out UCP2 as a key gene in inflammation by promoting immune cells activation and proliferation [3-6].
Post-doctoral work: 1993-1997 in John E Walker laboratory (Nobel Prize Winner Chemistry, 1997), Cambridge, Laboratory of Molecular Biology, MRC
Using a simple bacterial genetics approache, I derived T7 RNA polymerase bacterial expression hosts, C41(DE3) and C43(DE3), that are adapted to the large scale production of soluble and membrane proteins ([7], cited over 1000 times Google Scholar). In these mutant hosts, some membrane proteins trigger internal membrane proliferation opening the way to in vivo structural investigation of membrane proteins [8]. A large number of membrane proteins have been crystallized after overproduction in those bacterial strains.
PhD work: laboratory of Dr Daniel Ricquier (CEREMOD, Meudon, France) 1989-93
The Uncoupling Protein (UCP) from brown adipose tissue dissipates the mitochondrial proton electrochemical gradient. In order to understand the molecular basis of heat production by UCP, I have evidenced the first experimental topological model [9], a model confirmed ten years later by the first x-ray structure of the ADP/ATP exchangor [10].
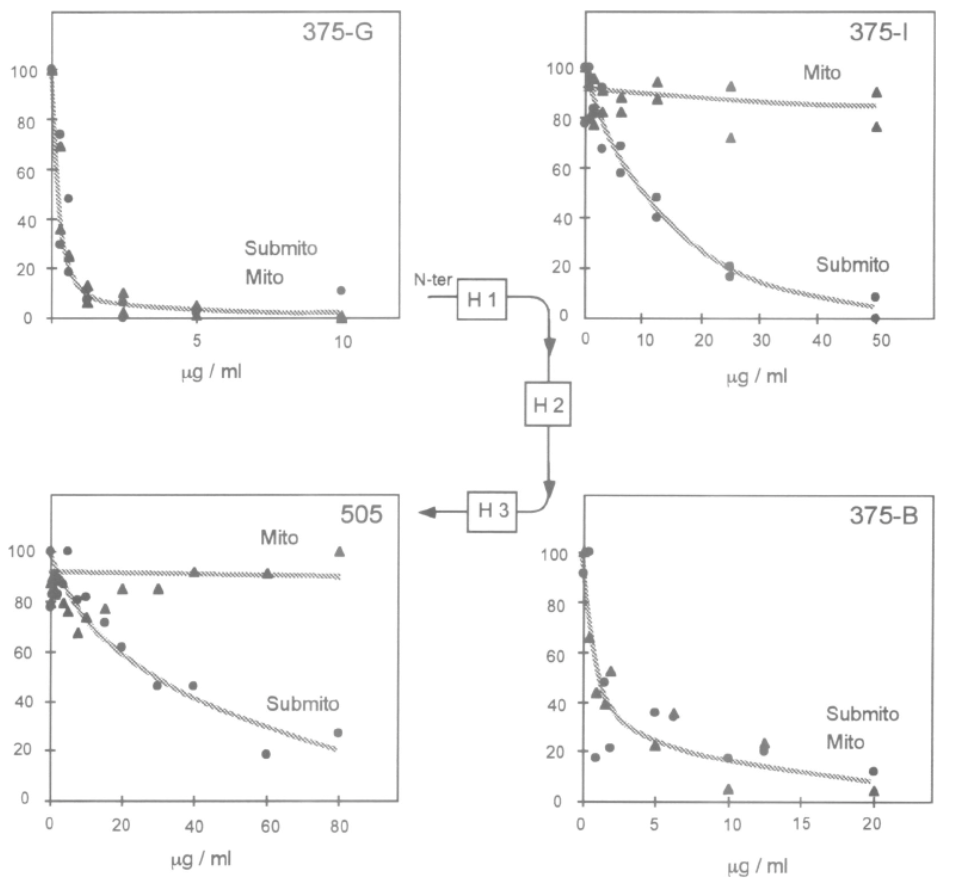
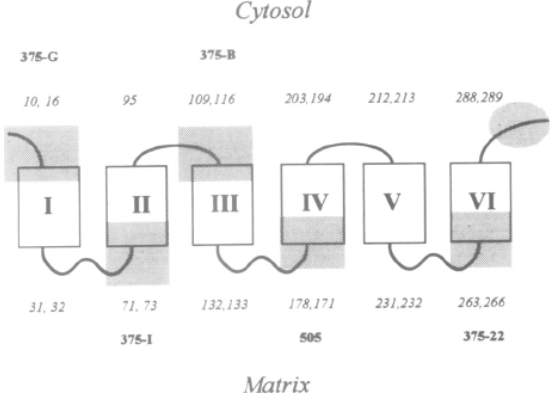
Adapted from [9]. Epitope specific anti-UCP1 antibodies were purified from 7 sheep sera [11]. Antibodies targeting UCP1 epitopes facing the cytosol can be titrated or trapped either by intact mitochondria or submitochondrial particles (see 375-G and 375-B antibodies). In contrast UCP1 antibodies against epitopes facing the matrix side of mitochondria are trapped only with submitochondrial particles. 375-G and 375-J epitopes have opposite topology thus demonstrating the presence of the H1 transmembrane span of UCP1.