Laurent J.
Catoire
Research Director

Miri
Garrigues
pHD STUDENT

Claudia
Zilian
Post-doc

Nahya
Nguyen Armengaud
PHD STUDENT
Ligand association to proteins constitutes a critical event in the activation or deactivation processes. Solution-state NMR can be amenable to high-resolution structure determination of various ligands in their protein-bound state by detecting dipolar interactions in a transferred mode. Based on observed dipolar interactions in NOESY spectra, it is possible to simultaneously determine, using a graphical approach, the auto (ρ) and cross (σ) relaxation rate constants in the bound state from 2D NOESY NMR experiments in the presence of chemical exchange [1].
Principle.
Both ρ and σ can be graphically estimated simultaneously from the experimental ratio of cross to diagonal peak volumes from 2D NOESY NMR experiments, Πexp, knowing the koff, the relative population of ligand vs. receptor, and ρ in the free state (see pdf.file).
Application.
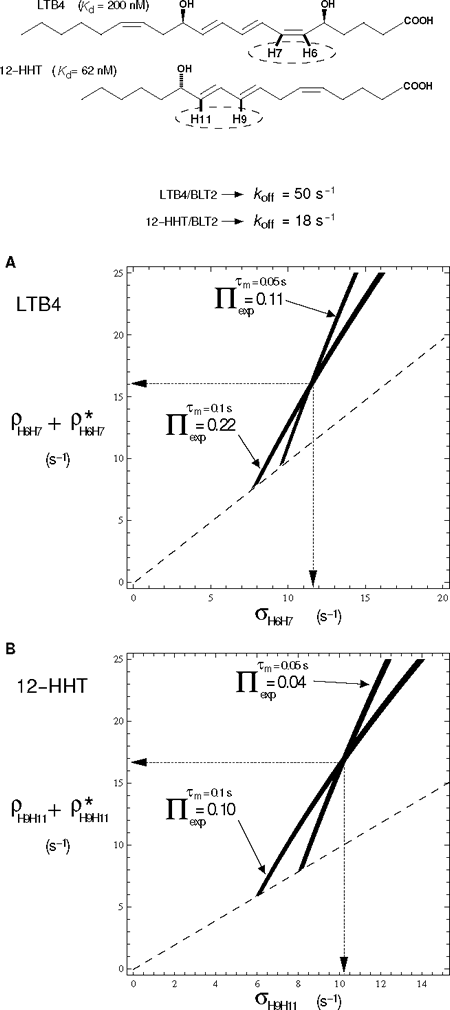
Fig. 1 illustrates two examples in the case of strong dipolar interactions, i.e. corresponding to short inter-proton distances in a rigid part of two unsaturated fatty acid compounds that are both agonists of the low affinity leukotriene G Protein-Coupled Receptor BLT2.
For further information, see ref 3.
Figure 1: Simultaneous graphical estimation of auto (ρ) and cross (σ) relaxation rate constants of LTB4 and 12-HHT in their BLT2-bound states in the presence of chemical exchange.
Top, chemical structures of LTB4 and 12-HHT. Dashed ellipses indicate the spin system for which ρ and σ relaxation are deduced from the graphic representations in A and B. Bottom, A and B correspond to superimposed projections along the cross to diagonal NOESY peak volume ratio, Π, axis of contour plots of theoretical Π taken at experimental Π values. NOESY volumes are measured at two mixing times (τm) (see corresponding LTB4/BLT2 and 12-HHT/BLT2 NOESY spectra in ref. 2 and 3, respectively). Illustration with the dipolar interactions between nuclei H6 and H7 of LTB4 (A) and H9 and H11 of 12-HHT (B) in the presence of ~9-fold excess of ligand over u-2H-BLT2. ρ* represents other non-dipolar relaxation contributions and/or a contribution from some other spins of the lattice. Contour lines are drawn for ρ + ρ* greater than or equal to σ (above the dashed line).
In collaboration with
Jean-Louis Banères
(IBMM, Montpellier)
(Marjorie Damian)
Eric Guittet
(ICSN, Gif/Yvette)
Marc Baaden
(IBPC, Paris)